SPA Meeting Reviews
Friday Session I: Opioids 1 – What is On Our (Bright) Horizon
What Neuroscience Says About the Strengths and Vulnerabilities of the Adolescent Brain
Reviewed by Sara Pittenger, MD
The University of North Carolina, Chapel Hill
 |
Dr. Pittenger |
This session outlined the following objectives:
- Understand the unique neurobiology of adolescence as a stage of brain development.
- Recognize the impact of adolescent neurobiology on brain health.
- Identify the impact of adolescent neurobiology on brain disorders, addiction, and injury.
Frances Jensen, MD (University of Pennsylvania Perelman School of Medicine) engaged the audience by outlining the underlying neurobiology that impact adolescent brain health and development of brain disorders and addiction. Be sure to refer to her numerous YouTube videos to learn more about her work and to help you better understand the adolescent brain that may live in your own home!
Dr. Jensen outlined some basic aspects of brain development; it is the last organ in the body to mature and maturation does not finish until mid to late 20s and demonstrates a gender difference. Myelination and synapse formation in the brain develops from “back to front” from the primitive limbic system to the prefrontal cortex which masters the executive function in the brain. Connectivity and excitation synapses peak in childhood and early adolescence and undergo pruning and creation of inhibitory impulses in later adolescence and adulthood. As such, the adolescent possessed a developed primitive limbic system without full maturation of connectivity to allow for inhibitory impulses from the higher functioning cortex. Dr. Jensen comically referred to the adolescent brain as “a Ferrari with weak brakes.”
Dr. Jenson demonstrated how the high level of excitatory synaptogenesis and plasticity in the young brain plays a crucial role in the ability to learn during this critical period of growth and development. At the neuronal level, learning occurs through the creation of a stimulus, the release of intracellular calcium, gene transcription and translation, and increased synaptic connectivity which later allows for higher long term potentiation and a stronger synaptic circuit. Because adolescent synaptic plasticity is superior to that of an adult, the adolescent demonstrates a stronger ability to learn and the ability to do so faster and more intensely.
With this fact, Dr. Jenson explained how increased synaptic plasticity in the adolescent brain translates to a stronger predisposition for addiction, when this learning occurs in primitive areas of the brain such as the hippocampus and nucleus accumbens. Because many medications and substances of abuse work on simple and complex synapses, their effect can be impacted by ongoing synapse formation of the individual exposed to these medications.
The adolescent brain is able to learn harder, faster and stronger, but is also able to become addicted harder, faster and stronger.
Looking specifically at the effect of various substances on the adolescent brain, Dr. Jenson highlighted the effects of nicotine, alcohol, opioids, and cannabis:
Nicotine exposure for the adolescent is actually increasing in the age of VAPE and e-cigarettes which can contain significantly more nicotine than an average cigarette. With this increased nicotine exposure, teenagers are responding more rapidly to the nicotine and it is changing the trajectory of their brain development, with slower development of the pre-frontal cortex.
Alcohol is a sedative that blocks synaptic plasticity at the level of the glutamate receptor and thus blunts learning. These effects can be long lasting or event permanent in the adolescent brain, with evidence showing adolescent alcohol exposure alters development of the frontal lobe.
Opioid use in the adolescent has been shown to be a growing problem. The enhanced neuronal learning in the adolescent brain results in an increased rate of addiction and the predisposal for refractory addiction in later adulthood. Opioid exposure in adolescence effects the frontal lobe which can manifest as an increased risk for depression.
Cannabis has the effect of blocking long term potentiation and synaptic plasticity by blocking intracellular signaling pathways. Effects have been shown to persist for several days and chronic daily exposure shows diminished long-term potentiation (and thus learning) even with discontinuation of use in adulthood. Data suggests that chronic daily cannabis use ages 13-20 can cause a dose-dependent decrease in IQ due to its effect on white mater development. There is also a potential association with adult psychosis/schizophrenia.
Dr. Jensen concluded her lecture discussing how differences in the adolescence brain can also explain vulnerability to not only addiction, but also to psychiatric disease states during this time. The adolescent is exposed to tremendous stress and social anxiety, and their brain processes stress differently than the child or adult brain, with an enhanced response to fearful stimuli. These factors contribute to prevalence of new onset of mental illness in adolescence, where an enhanced stress response and an immature frontal cortex predispose the at risk adolescent to the development of mental illness.
In conclusion:
Adolescent brains have different synaptic physiology and neurochemistry
- Expression and function differences not just of the opioid system but also non-opioid therapeutic targets
- Biology suggest a very age-specific strategy
- Synaptic plasticity that underlies addiction is more active is adolescents that adult, and risk behavior is relatively higher
Long term changes are seen with all substances of abuse
-
Particularly (ironically) affecting the impulse control circuitry in the prefrontal areas that are still developing
Abuse Deterrent Opioids: The Answer to an Epidemic? Or Wolves in Sheep’s Clothing
This session outlined the following objectives:
- Learn the theory behind why abuse deterrent opioids have potential to decrease opioid abuse and addiction
- List the different abuse deterrent formulations (ADFs) and their pharmacologic profiles
- Describe the barriers to ADF use and their unintended consequences with regard to the fight against the opioid epidemic
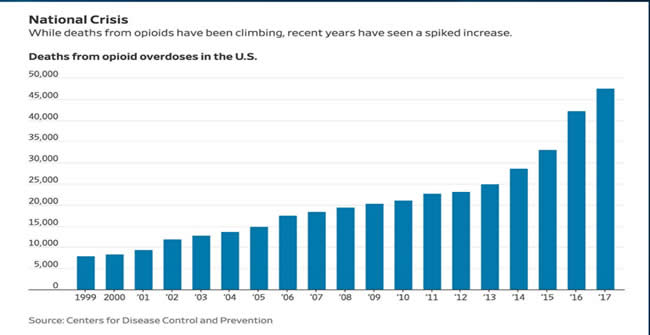
Ronald Litman, DO, FAAP (Children’s Hospital of Philadelphia, Medical Director for Institute of Safe Medication Practices) began his talk identifying our national opioid crisis and the rising number of opioid overdose-related deaths over the 20 years. He emphasized the relevance of this issue for our pediatric patients as demonstrated in a recent publication by Harbaugh CM, et al in JAMA Surg Feb 2019 entitled “Association Between Long-term Opioid Use in Family Members and Persistent Opioid Use After Surgery Among Adolescents and Young Adults”. Here, children with family members with long-term opioid use were twice as likely to have a postoperative opioid medication refill than patients without family-level risk factors.
Dr. Litman polled the audience on their knowledge of Abuse Deterrent Opioid Formulations and their opioid prescribing practices. Nearly 50% of the audience was not familiar with ADFs and less than 10% has ever prescribed these medications.
Abuse deterrent formulations (ADFs) are opioid formulations manufactured with the intention of reducing the abuse and misuse of these medications. The first ADF in formulation was Oxycontin, manufactured in 1995 with the intention of having decreased abuse potential than the immediate release formulation. The FDA requires medications identified as ADFs to be scientifically proven to deter one type of abuse: chewing, snorting, inhaling, or intravenous administration. Other forms of ADFs include opioid agonist/antagonist combinations including naltrexone or naloxone. Future possible technologies for ADF formulations may include aversion methods such as nasal irritation when snorted, SubQ implants of opioid formulations, and Pro drugs.
Dr. Litman outlined the FDA testing requirements necessary for a medication to be labeled as an abuse deterrent opioid formulation:
- Category 1 – laboratory-based in vitro manipulation and extraction studies
- Category 2 – human pharmacokinetic studies
- Category 3 – clinical abuse potential studies – these are controlled studies with known recreational opioid abusers to determine the likability and abuse potential of the medication
- Category 4 – Post marketing studies to assess the impact of an ADF on actual abuse. Two major programs exist, National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) and Diversion and Addiction-Related Surveillance (RADARS) to look at medication use, abuse, and safety once the drug is in the market
There are currently eight approved ADFs, with only the first five (*) available in the US:
- OxyContin (Oxycodone ER)*
- Embeda (Morphine + Naltrexone)*
- Hysingla ER (Hydrocodone)*
- MorphaBond ER (Morphine)*
- Xtampza ER (Oxycodone)*
- Arymo ER (Morphine)
- RoxyBond (IR Oxycodone)
- Targinq ER (Oxycodone + Naloxone)
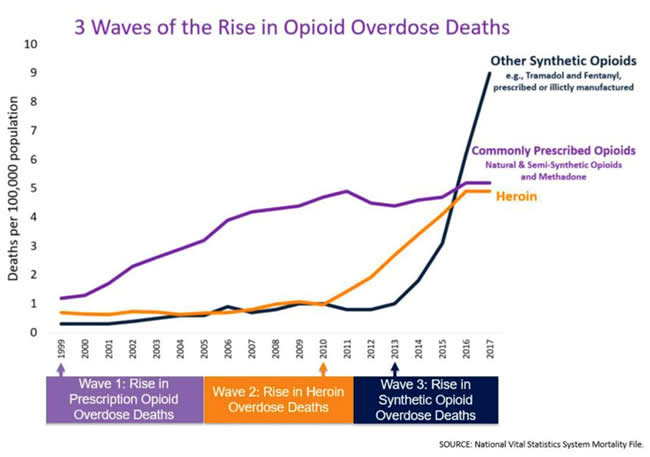
Dr. Litman continued by asking the provocative question of whether ADFs work at addressing the opioid epidemic, or if they are instead “Wolf in sheep’s clothing.” He showed prescription trends of common oral opioids, with OxyContin having the lowest rate when compared to immediate release formulations, and identified that the majority of deaths from prescription opioids are due to overdose from taking too many pills as opposed to overdose from altering the medication to inhale, snort or inject. He also returned to the previously referenced graph (see above) which demonstrates three waves of rise in opioid deaths. Wave 1 is a rise in prescription opioid overdose deaths after the advent of ADFs. Wave 2 and 3 however, show a rise in heroin and fentanyl, respectively, thus suggesting that abusers are turning to different medications as prescription opioids are becoming more difficult to obtain and alter for recreational use and abuse. He also presented CDC Data showing rise in cocaine and methamphetamines after the creation of ADFs.
Dr. Litman highlighted that four out of five heroin users first become addicted to prescription opioids, suggesting that preventing first opioid use may have longer standing impact on preventing heroin addiction and the opioid epidemic.
Dr. Litman also highlighted current political and financial influences related to ADFs, with progressive legislature in place to move towards promotion of ADFs and pharmaceutical lobbyists working to pass laws to promote ADFs. He identified that some insurance companies are also moving to only reimbursing ADFs and not standard opioid formulations, with questionable impact on overall opioid abuse and misuse.
He closed his lecture by identifying three patient populations that may be differentially impacted by ADFs:
- Chronic Pain Patient – these patients are less likely to misuse or divert opioids. ADFs may be more costly to obtain than standard opioid formulations
- Severe Opioid User – ADFs are more likely to cause harm due to health consequences from using ADFs improperly and the increased likelihood of transition to heroin
- Acute pain patient/novice or recreational use – these patients are at an increased risk for diversion or misuse. ADFs in this population may deter initiation of misuse or abuse of opioids. This population is the most likely group to benefit
New Targets for Pain Management: B-arrestin Ligands, Kappa antagonists and peripheral opioid antagonists? These ain’t no Drank, Dilly, or Oxycotton
This session outlined the following objectives:
- Review issues with current opioid analgesics
- Recognize emerging analgesic targets
- Evaluate new pain medications based on their mechanisms and side effects
Thomas A. Anderson, MD, PhD (Lucile Packard Children’s Hospital Stanford) first engaged the audience through the audience response system to see who was similar with “Purple Drank,” which is the name given to a recreational drug created with codeine-containing cough medicine, soft drink, and a jolly rancher.
Dr. Anderson began his talk outlining some of the concerns with currently available opioid analgesics including poor pain management for chronic pain patients and numerous acute and chronic side effects. He referenced a study by Long DR in the British Journal of Anaesthesia 2018 showing higher intraoperative opioid use had higher 30-day rate of readmission. He also referenced an article by Harbaugh CM et al in Pedaitrics 2018 showing the persistent opioid use was present in 4.8% of pediatric patients 3-6 months after surgery where opioids were prescribed post operatively.
Dr. Anderson explained that a large number of target receptors are being identified for analgesic effect and side effect reduction. Opioids work at the mu-opioid receptor (MOR), a seven-transmembrane domain G protein-coupled receptors (GPCR). Multiple MOR subtypes exist (mu, delta, kappa, sigma). Simply put, MOR GPCRs take extracellular signals and internalize them into the cells resulting is a broad signaling cascade. Novel opioid molecules are able to selectively bind these receptors thus resulting in more specific pathway activation.
Dr. Anderson discussed the target development of novel opioid therapies is focused on biased ligands – medications that can be developed to specifically activate the G protein pathway, without unwanted pathway activation (such as B-arrestin pathway) and this fewer unwanted side effects.
Dr. Anderson highlighted some example novel opioid therapies in early development, the first being TRV130. This is a mu-opioid ligand with G-proteon coupling similar to morphine but with decreased receptor phosphorylation, decreased beta-arrestin engagement and decreased internalization. In preliminary studies, TRV130 has demonstrated improved analgesia when compared to morphine and decreased respiratory depression and nausea. It also appears to lack rapid development of tolerance. Larger clinical trial needed before FDA approval.
Other targets for novel agents include medications that target a specific MOR. Selective agonists and antagonists of kappa opioid receptors have been investigated. Biased Kappa opioid receptor agonists have shown promising results for the treatment of pain and intractable itch. Selective agonist/antagonists might be used to treat cocaine addiction, depression, feeding behavior, psychosis and schizophrenia. One specific KOR agonist, CR 845, is in the early trial phases.
Other examples of novel strategies include the endomorphin 1 analogue CYT-1010, which targets the mu opioid receptor and has recently been approved to move forward with phase 2 trials. NKTR-181 is a new molecular entity mu opioid agonist by Nektar therapeutics designed to provide analgesia and reducing abuse potential due to its specific binding and limited penetrance of the blood brain barrier. NKTR-181 is undergoing phase 3 randomized controlled trials looking at efficacy for treating chronic pain.
The final category of novel opioid therapies presented by Dr. Anderson were peripheral opioid antagonists. These agents target the mu opioid receptor peripherally without reversing analgesia. Three such agents are currently approved by the FDA for treatment of opioid-induced constipation and postoperative ileus:
- Alvimopan – improves ileus and decreases LOR and ileus-related readmission after intestinal resection
- Methnaltrexone – effective treatment of opioid constipation
- Naloxegol – shown to increase bowel movements in patients with non-cancer pain and opioid-induced constipation
In conclusion, current analgesics, especially opioids are associated with a myriad of serious adverse events. Emerging analgesics are selective or take advantage of biased agonist potential of G-protein coupled receptors to provide analgesia while reducing opioid-related adverse events.






